Medical Mask Standards
The Science Behide Our Most Trusted Shield
In hospitals and operating rooms, every piece of equipment plays a vital role in protecting both patients and healthcare workers.
Among these tools, the medical face mask — or surgical mask — stands as one of the most visible yet most scientifically tested barriers.
It’s not just a piece of fabric covering the mouth and nose; it’s a certified medical device carefully engineered to act as a frontline defense.
A medical mask serves two crucial purposes:
1️⃣ To protect the wearer from splashes of bodily fluids such as blood or secretions that may contain pathogens.
2️⃣ To prevent respiratory droplets from the wearer from contaminating patients or sterile environments.
But how can we be sure that these masks truly perform as claimed?
The answer lies not in how they look, but in the rigorous scientific testing they must pass under internationally recognized standards.
This article explores how these global standards ensure safety — and the essential role of synthetic blood simulants in proving that protection works under real surgical conditions.
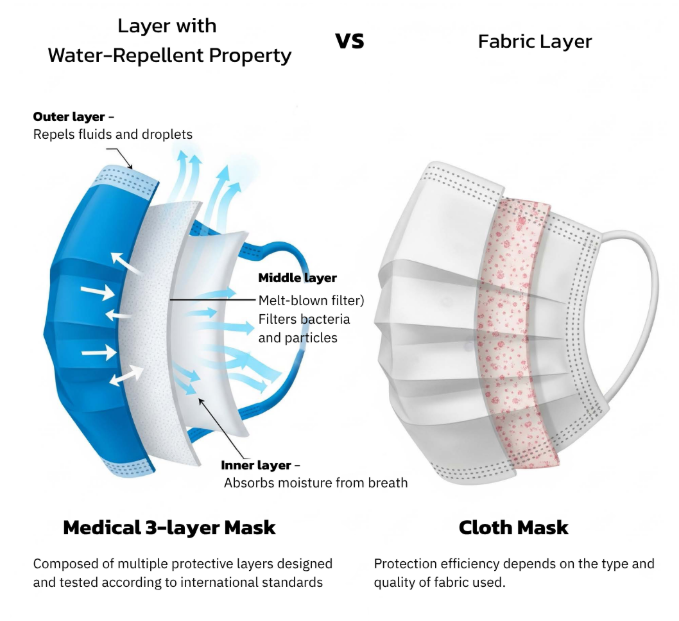
The Dual Protection of Medical Masks
Global Safety Frameworks: Understanding ASTM F2100 and EN 14683
To ensure consistency and safety across manufacturers worldwide, international organizations have established clear testing protocols.
Two major standards are widely recognized:
- ASTM F2100 (USA)
Developed by ASTM International (American Society for Testing and Materials), this standard classifies masks into three performance levels:
- Level 1 – Low Risk: For general use where minimal exposure to fluids or droplets is expected.
- Level 2 – Moderate Risk: For procedures with moderate fluid exposure, such as emergency rooms or dental clinics.
- Level 3 – High Risk: For high-pressure fluid environments, such as major surgeries or trauma care.
- EN 14683 (Europe)
Issued by the European Committee for Standardization (CEN), this standard categorizes masks into Type I, Type II, and Type IIR, where the letter “R” denotes resistance to liquid splashes
The Thai Standard: From TIS 2424-2562 to TIS 2424-2565
In Thailand, medical masks are regulated as medical devices by the Thai FDA and must comply with the Thai Industrial Standard (TIS).
The earlier standard, TIS 2424-2562, was officially replaced by TIS 2424-2565: Disposable Medical Masks, effective 120 days after its publication in the Royal Gazette on January 11, 2023.
The revision was designed to align Thai standards with global norms — particularly ASTM F2100 — ensuring fair trade, smooth exports, and consistency across diverse mask designs available in the market.
Today, the Department of Medical Sciences (Ministry of Public Health) provides official testing services for masks under this updated standard.
So, if you still see “TIS 2424-2562” on a box — don’t worry. Those masks were certified under the previous regulation and remain valid.
Ultimately, these standards guarantee that the masks we use — whether in hospitals or everyday life — have passed critical scientific assessments to prove their protective quality
Comparative Overview: ASTM F2100 vs EN 14683
|
Test |
ASTM F2100 Level 1 |
ASTM F2100 Level 2 |
ASTM F2100 Level 3 |
EN 14683 Type II |
EN 14683 Type IIR |
|
Bacterial Filtration Efficiency (BFE) |
≥95% |
≥98% |
≥98% |
≥98% |
≥98% |
|
Particle Filtration Efficiency (PFE) @ 0.1 µm |
≥95% |
≥98% |
≥98% |
ไม่กำหนด |
ไม่กำหนด |
|
Fluid Resistance (Pressure) |
80 mmHg |
120 mmHg |
160 mmHg |
ไม่กำหนด |
≥120 mmHg (16.0 kPa) |
|
Differential Pressure (ΔP) |
<5.0 mmH₂O/cm² |
<6.0 mmH₂O/cm² |
<6.0 mmH₂O/cm² |
<40 Pa/cm² |
<60 Pa/cm² |
|
Flammability |
Class 1 |
Class 1 |
Class 1 |
ไม่กำหนด |
ไม่กำหนด |
Passing the Test: The Five Core Performance Evaluations
To earn certification, a medical mask must pass five critical tests, each designed to simulate a real-world challenge.
1. Bacterial Filtration Efficiency (BFE)
This test measures how effectively the mask filters out bioaerosols containing bacteria — simulating what happens when someone coughs or sneezes.
A fine mist containing Staphylococcus aureus (average particle size: 3.0 µm) is sprayed through the mask, and the number of bacteria that pass through is counted using a Cascade Impactor. High-performance masks must achieve BFE ≥ 98%.
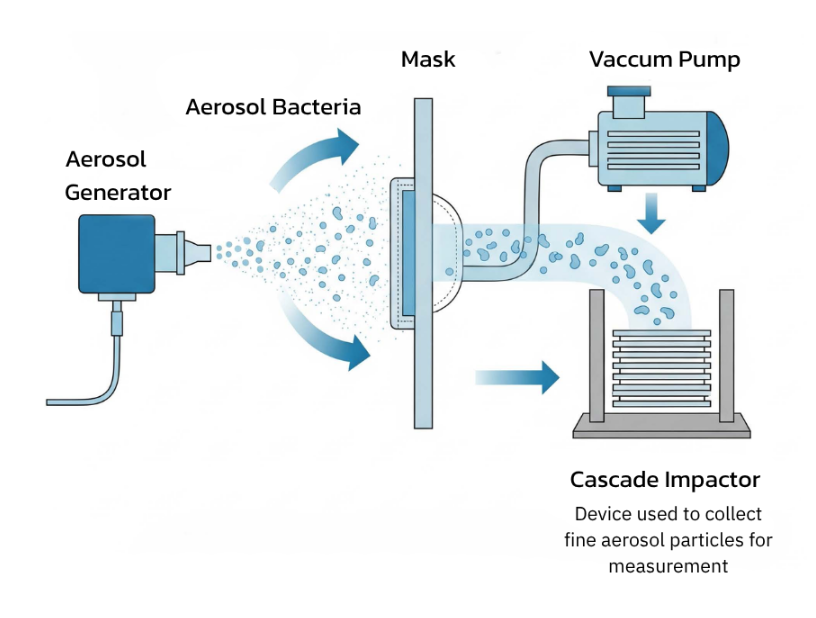
Bacterial Filtration Efficiency (BFE) testing setup.
2. Particle Filtration Efficiency (PFE)
PFE testing is even more demanding.
It measures the ability to filter submicron particles, typically 0.1 µm latex spheres — roughly the size of some viruses.
This test is required under ASTM F2100 but optional in EN 14683, highlighting the stricter U.S. criteria for fine-particle filtration.
3. Differential Pressure (ΔP): Breathability
This test evaluates how easy it is to breathe through the mask.
Air is passed through the mask at a constant flow rate of 8 L/min, and resistance is measured.
A lower ΔP value means easier breathing; too high, and the mask may cause discomfort or leakage around the edges.
Thus, standards specify maximum limits to balance filtration efficiency and comfort.
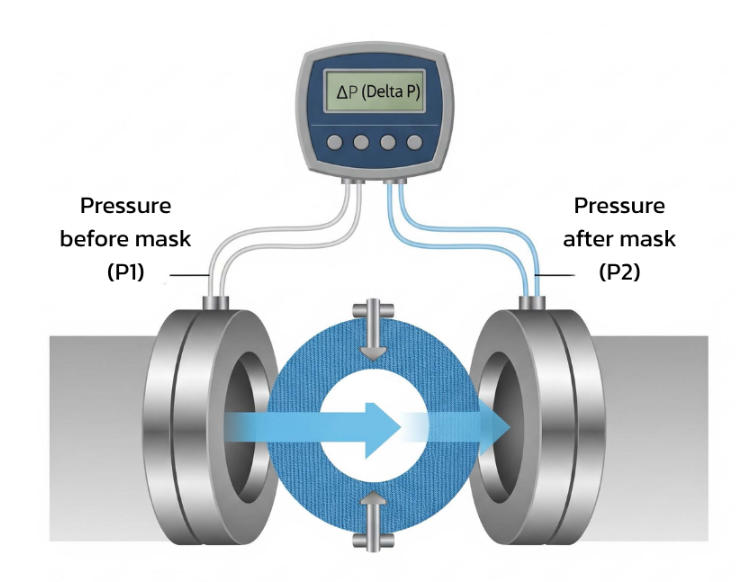
Differential pressure testing apparatus — measuring breathability through the mask.
4. Flammability
Operating rooms can contain oxygen-enriched environments and ignition sources such as electrocautery tools.
To prevent fire hazards, masks are tested under 16 CFR Part 1610, where a sample is exposed to flame for 1 second.
It must self-extinguish quickly to meet Class 1 flammability.
5. Fluid Resistance: The Blood Splash Test
Perhaps the most crucial test of all — this one simulates a high-pressure splash of blood or bodily fluid striking the mask surface and at the center of this evaluation lies the unsung hero: synthetic blood.
Beyond the Basics: Additional Biocompatibility Tests
For high-protection masks (Level 3), skin irritation and sensitization tests are required to ensure that prolonged contact with the skin is safe.
These are part of the broader biocompatibility assessments — confirming that materials won’t cause rashes, redness, or allergic reactions
Spotlight: The Synthetic Blood Penetration Test (ASTM F1862 / ISO 22609)
This test is designed to simulate the worst-case scenario in surgery — when a blood vessel is cut and high-pressure blood jets toward the mask.
How it works:
A mask sample is mounted vertically. A pneumatic system then fires 2 mL of synthetic blood through a small cannula from a distance of 30.5 cm at controlled velocity — mimicking real blood pressure conditions:
- 80 mmHg: Venous pressure (ASTM Level 1)
- 120 mmHg: Arterial pressure (ASTM Level 2)
- 160 mmHg: Trauma-level pressure (ASTM Level 3)
After the spray, the inner side of the mask is visually inspected. If no penetration is observed, the mask passes at that pressure level.
To meet FDA requirements, 32 samples must be tested,
and at least 29 must pass — meeting the Acceptable Quality Level (AQL) of 4.0%.
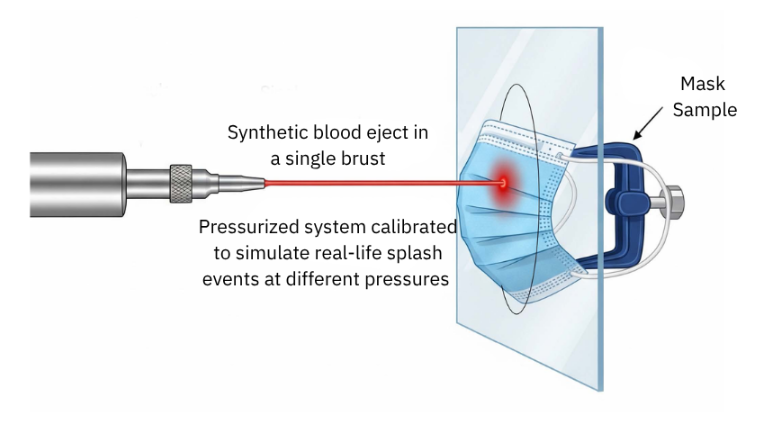
Synthetic Blood Penetration Test setup — simulating real surgical splash conditions.
The Heart of the Test: Why Synthetic Blood Must Meet Standards
The reliability of this test depends entirely on the quality of the synthetic blood used. Using regular colored water would render the results meaningless — or worse, allow unsafe masks to pass certification.
A compliant synthetic blood must replicate key physical properties of real human blood:
- Surface Tension: Perhaps the most critical factor. Human blood’s surface tension ranges between 42–60 dynes/cm. ASTM F1862 specifies 40 ± 5 dynes/cm to replicate the most challenging conditions for mask penetration. Liquids with higher surface tension won’t penetrate as easily, producing falsely “good” results.
- Viscosity and Density: Viscosity is adjusted with thickeners so that the liquid flows like real blood. Density is maintained at around 1.005 g/cm³ to ensure the correct kinetic energy and impact force during spraying.
If these parameters vary between batches, results become unreliable. Hence, lot-to-lot consistency and certified quality control are essential for trustworthy outcomes. Certified synthetic blood must match human blood’s surface tension, viscosity, and density to ensure valid test results.
"At IRPC Innovation Center, our team has developed synthetic blood formulations that meet ASTM F1862 criteria in every batch — ensuring accurate, reproducible, and internationally recognized PPE testing results."
Final Reflection: Trust Built on Standards and Science
A medical mask is more than just a product — it’s a promise of safety for frontline healthcare workers and every patient they protect. That promise is only real when supported by rigorous testing under trusted global standards.
Among all the evaluations, the synthetic blood penetration test stands as the ultimate trial, replicating the most dangerous real-world scenarios.
The credibility of this test depends directly on the quality of the blood simulant used.
If the fluid doesn’t meet standards, the certification becomes meaningless — and lives could be at risk.
That’s why hospitals, procurement teams, and mask manufacturers must treat testing standards and certified materials as long-term investments in safety and reliability.
Together, they form a Chain of Trust — from the testing lab to the production line, all the way to the people who rely on these masks every day.
If you’d like more information about certified synthetic blood for mask testing — or expert guidance on improving your quality control process — our team is ready to help.
Work cited
- Healthcare Respiratory Protection - CDC, accessed August 6, 2025
- N95 Respirators, Surgical Masks, Face Masks, and Barrier Face Coverings | FDA, accessed August 6, 2025
- Strategies for Conserving the Supply of Medical Masks | Healthcare Workers - CDC, accessed August 6, 2025
- Standard for Medical Face Masks, ASTM F2100-23 - The ANSI Blog, accessed August 6, 2025
- Guide to Face Mask Selection and Use, accessed August 6, 2025
- MEDICAL FACE MASK TESTS AND REQUIREMENTS | Nelson Labs, accessed August 6, 2025
- Medical face masks - Requirements and test methods BS EN 14683:2019 - LISUN, accessed August 6, 2025
- กรมวิทยาศาสตร์การแพทย์ ให้บริการทดสอบหน้ากากอนามัยทางการแพทย์, accessed August 6, 2025
- มาตรฐานผลิตภัณฑ์อุตสาหกรรม มอก. 2424-2562 หน้ากากอนามัย - BDS, accessed August 6, 2025
- ก.อุตฯ เข้มมาตรฐาน “หน้ากากอนามัยใช้ครั้งเดียว - กระทรวงอุตสาหกรรม, accessed August 6, 2025
- BS EN 14683:2019 - Medical Face Mask - Requirements and test methods - Microbe Investigations, accessed August 6, 2025
- Bacterial Filtration Efficiency (BFE) - SIG LABORATORY, accessed August 6, 2025
- ASTM F2100 - Performance of materials used in medical face masks, accessed August 6, 2025
- Surgical Face and General-Use Masks Tests - Nelson Labs, accessed August 6, 2025
- F1862/F1862M Standard Test Method for Resistance of Medical Face Masks to Penetration by Synthetic Blood (Horizontal Projection of Fixed Volume at a Known Velocity) - ASTM, accessed August 6, 2025
- ASTM F1862-05 - iTeh Standards, accessed August 6, 2025
- Synthetic Blood for PPE Testing - Kinectrics, accessed August 6, 2025
- The Surface Tension of Synthetic Blood used for ASTM F1670 Penetration Tests - PMC, accessed August 6, 2025
- F1862/F1862M Standard Test Method for Resistance of Medical Face Masks to Penetration by Synthetic Blood (Horizontal Projection of Fixed Volume at a Known Velocity) - ASTM, accessed August 6, 2025
- Synthetic Blood Penetration Testing: Using Fluid Dispensing Solutions to Determine Surgical Mask Effectiveness | Nordson EFD, accessed August 6, 2025
